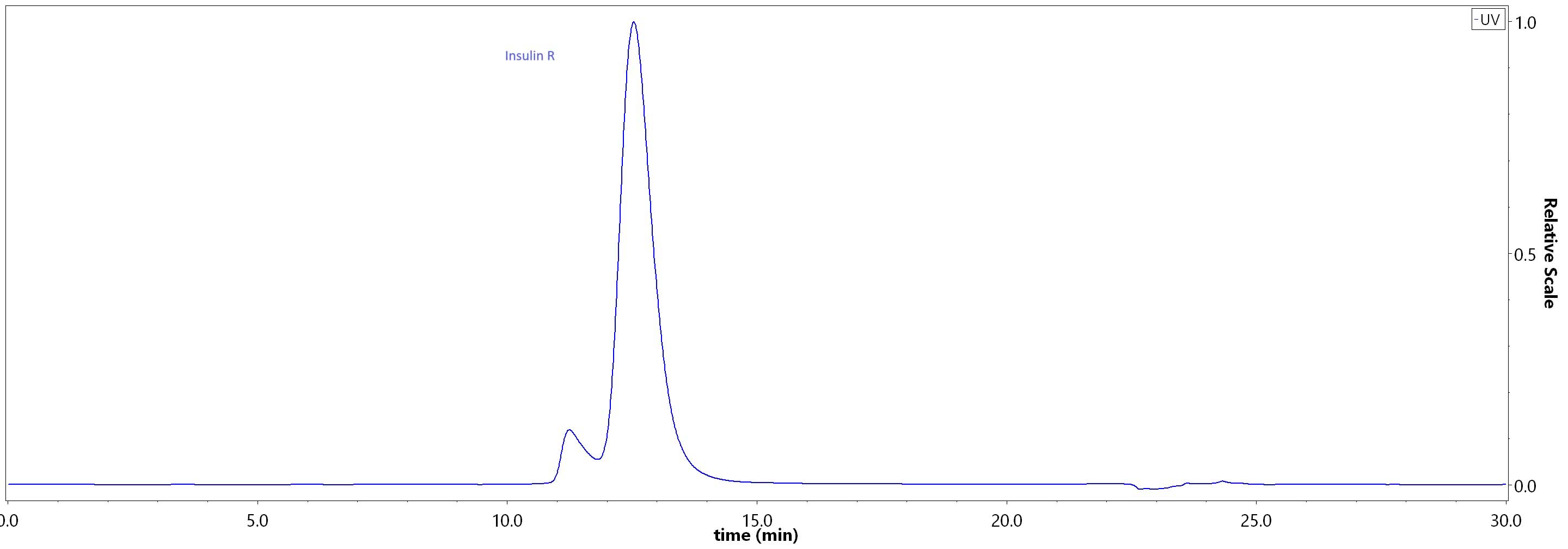
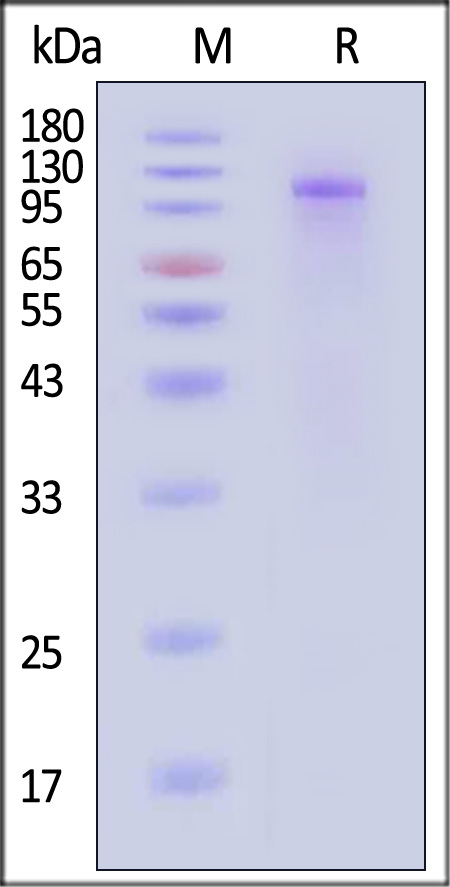
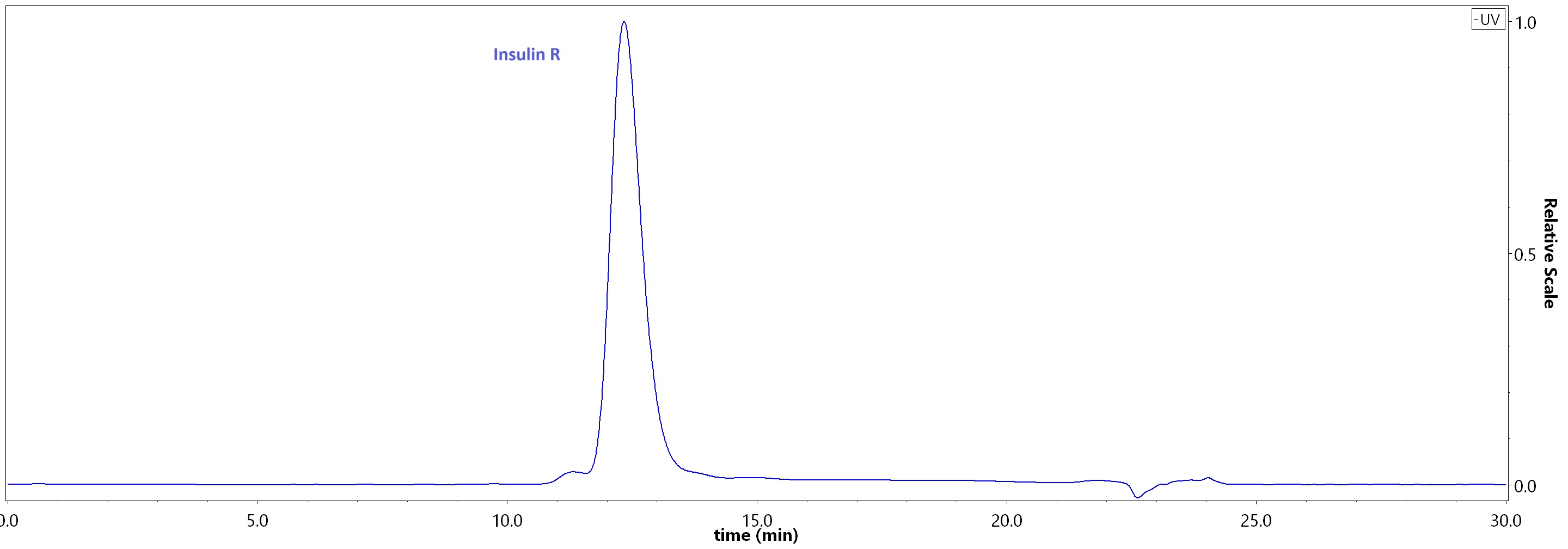
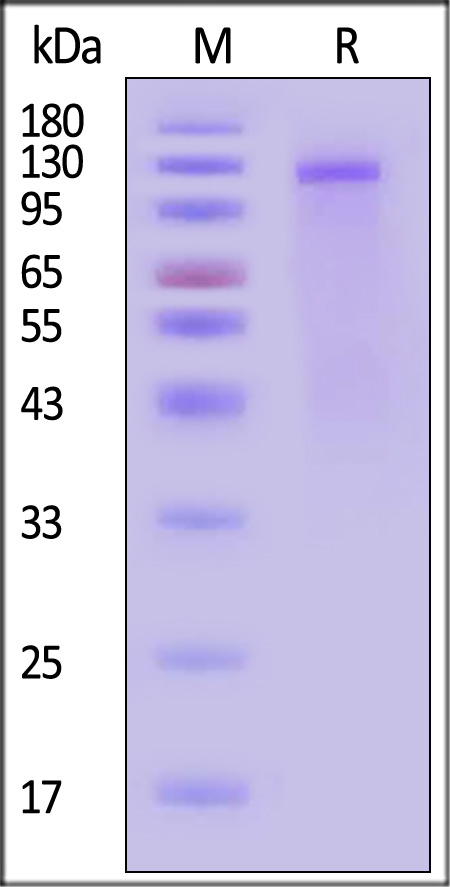
| Isophane Protamine Recombinant Human Insulin Injection (Shenzhen Kexing Biotech) |
精蛋白重组人胰岛素注射液(深圳科兴) |
|
批准上市 |
深圳科兴生物工程有限公司 |
苏泌啉恩 |
Mainland China |
糖尿病 |
Shenzhen Kexing Biological Engineering Co Ltd |
2003-02-24 |
糖尿病 |
详情
|
| Insulin glargine biosimilar (Tonghua Dongbao Pharmaceutical) |
甘精胰岛素生物类似药(通化东宝) |
|
批准上市 |
通化东宝药业股份有限公司 |
长舒霖 |
Mainland China |
糖尿病 |
Tonghua Dongbao Pharmaceutical Co Ltd |
2019-12-06 |
二型糖尿病, 糖尿病 |
详情
|
| Human insulin biosimilar (Square Pharmaceuticals) |
人胰岛素生物类似药 (Square Pharmaceuticals) |
|
批准上市 |
Square Pharmaceuticals Ltd |
Ansulin |
Bangladesh |
糖尿病 |
Square Pharmaceuticals Ltd |
2013-01-01 |
糖尿病 |
详情
|
| Recombinant human insulin (United Laboratories International Holdings) |
|
|
批准上市 |
珠海联邦制药股份有限公司中山分公司 |
优思灵, Uslin, 优思灵R |
Mainland China |
糖尿病 |
Zhongshan Branch of Zhuhai United Laboratories Co Ltd |
2009-11-25 |
糖尿病 |
详情
|
| Insulin glargine biosimilar (Getz Pharma) |
甘精胰岛素生物类似药 (Getz Pharma) |
|
批准上市 |
Getz Pharma |
Basagine |
|
|
|
|
一型糖尿病, 二型糖尿病 |
详情
|
| Recombinant human insulin (Tonghua Dongbao Pharmaceutical) |
重组人胰岛素(通化东宝) |
|
批准上市 |
通化东宝药业股份有限公司 |
甘舒霖, 甘舒霖R |
Mainland China |
糖尿病 |
Tonghua Dongbao Pharmaceutical Co Ltd |
1998-01-01 |
糖尿病 |
详情
|
| Insulin aspart |
门冬胰岛素 |
NN-1218; INA-X14; Insulin X14 |
批准上市 |
诺和诺德制药 |
Novolog, NovoRapid, NovoLog FlexPen, NovoMix, Fiasp, 诺和锐, NovoRapid/NovoLog |
EU |
糖尿病 |
Novo Nordisk A/S |
1999-09-07 |
一型糖尿病, 二型糖尿病, 动脉粥样硬化, 谵妄, 高胆固醇血症, 高血压, 术后认知障碍并发症, 冠状动脉疾病, 轻度认知障碍, 阿尔茨海默病, 心脏和血管疾病, 糖尿病 |
详情
|
| Insulin glargine biosimilar (LG Life Sciences) |
甘精胰岛素生物类似药 (LG Life Sciences) |
|
批准上市 |
Lg生命科学 |
Basugine |
|
|
|
|
一型糖尿病, 二型糖尿病 |
详情
|
| Insulin biosimilar (Wockhardt) |
胰岛素生物类似药 (Wockhardt) |
|
批准上市 |
Wockhardt Ltd |
Wosulin Pen Royale, Wosulin, Wosulin®-R, Wosulin Pen |
India |
一型糖尿病 |
Wockhardt Ltd |
2003-10-01 |
一型糖尿病, 糖尿病 |
详情
|
| Insulin detemir |
地特胰岛素 |
NN-304 |
批准上市 |
诺和诺德制药 |
诺和平, Levemir |
EU |
糖尿病 |
Novo Nordisk A/S |
2004-06-01 |
一型糖尿病, 二型糖尿病, 动脉粥样硬化, 高胆固醇血症, 高血压, 妊娠期糖尿病, 冠状动脉疾病, 阿尔茨海默病, 轻度认知障碍, 心脏和血管疾病, 糖尿病 |
详情
|
| Insulin biosimilar (Popular Pharmaceuticals) |
胰岛素生物类似药 (Popular Pharmaceuticals) |
|
批准上市 |
Popular Pharmaceuticals |
Insul R |
Bangladesh |
糖尿病 |
Popular Pharmaceuticals |
2013-01-01 |
糖尿病 |
详情
|
| Insulin degludec/Liraglutide |
德谷胰岛素/利拉鲁肽 |
NN-9068 |
批准上市 |
诺和诺德制药 |
Xultophy, iDegLira |
EU |
二型糖尿病 |
Novo Nordisk A/S |
2014-09-18 |
二型糖尿病, 糖尿病 |
详情
|
| Imeglimin hydrochloride |
|
EMD-387008; PXL-008 |
批准上市 |
德国默克公司 |
Twymeeg, ツイミーグ |
Japan |
二型糖尿病 |
Poxel Sa, Sumitomo Dainippon Pharma Co Ltd |
2021-06-23 |
二型糖尿病, 心脏病, 肝功能衰退 |
详情
|
| Insulin biosimilar (Sedico) |
胰岛素生物类似药 (Sedico) |
|
批准上市 |
Sedico |
Insulin H bio R |
Egypt |
糖尿病 |
Sedico |
2013-02-01 |
糖尿病 |
详情
|
| Insulin aspart biosimilar (Biocon/Mylan) |
门冬胰岛素生物类似药 (Biocon/Mylan) |
MYL-1601D |
批准上市 |
Biocon Ltd |
|
EU |
糖尿病 |
Biosimilar Collaborations Ireland Ltd |
2021-02-05 |
糖尿病 |
详情
|
| Insulin human (rDNA origin, Lilly) |
基因重组人胰岛素(礼来) |
U-500R; S-3300; LY-041001 |
批准上市 |
礼来制药 |
Humulin R Kwikpen, Humulin, Humulin R, 优泌淋R, Humulin R Pen |
United States |
二型糖尿病 |
Eli Lilly And Company |
|
一型糖尿病, 二型糖尿病, 脑癌, 阿尔茨海默病, 轻度认知障碍, 颅内动脉瘤, 糖尿病 |
详情
|
| Insulin glargine biosimilar (Wockhardt) |
甘精胰岛素生物类似药 (Wockhardt) |
|
批准上市 |
Wockhardt Bio Ag |
Glaritus, Glaritus Cart, Glaritus DispoPen |
India |
糖尿病, 一型糖尿病, 二型糖尿病 |
Wockhardt Ltd |
2013-01-01 |
一型糖尿病, 二型糖尿病, 糖尿病 |
详情
|
| Insulin aspart biosimilar (Tonghua Dongbao Pharmaceutical) |
门冬胰岛素生物类似药(通化东宝) |
|
批准上市 |
通化东宝药业股份有限公司 |
|
Mainland China |
糖尿病 |
Tonghua Dongbao Pharmaceutical Co Ltd |
2021-10-19 |
二型糖尿病, 糖尿病 |
详情
|
| Human insulin biosimilar (MJ Biopharm) |
人胰岛素生物类似药 (MJ Biopharm) |
|
批准上市 |
Mj Biopharm Pvt |
Biosulin |
India |
糖尿病 |
Mj Biopharm Pvt |
2005-12-01 |
糖尿病 |
详情
|
| Insulin glargine biosimilar(Shandong New Time Pharmaceutical) |
重组甘精胰岛素生物类似药(山东新时代药业) |
|
批准上市 |
鲁南制药集团股份有限公司 |
|
Mainland China |
糖尿病 |
Shandong New Time Pharmaceutical Co Ltd |
2022-05-10 |
糖尿病 |
详情
|
| Insulin lispro biosimilar (Sanofi) |
赖脯胰岛素生物类似药(赛诺菲) |
SAR-342434 |
批准上市 |
赛诺菲 |
Admelog |
EU |
糖尿病 |
Sanofi Winthrop Industrie SA |
2017-07-18 |
一型糖尿病, 二型糖尿病, 糖尿病 |
详情
|
| Insulin biosimilars (Bioton) |
胰岛素生物类似药 (Bioton) |
|
批准上市 |
Bioton Sa |
|
Mainland China |
糖尿病 |
Bioton Sa |
2013-01-01 |
糖尿病 |
详情
|
| Insulin glargine biosimilar (Biocon) |
甘精胰岛素生物类似药 (Biocon) |
MYL-1501D |
批准上市 |
富士胶片株式会社, Biocon Biopharmaceuticals, Mylan Nv |
Semglee, Basalog |
India |
糖尿病 |
null |
2009-01-01 |
一型糖尿病, 二型糖尿病, 糖尿病 |
详情
|
| Insulin glargine biosimilar (Wanbang Biochemical) |
重组甘精胰岛素生物类似药(万邦医药) |
|
批准上市 |
江苏万邦生化医药集团有限责任公司 |
|
Mainland China |
糖尿病 |
Wanbang Biopharmaceuticals Co Ltd |
2022-08-30 |
二型糖尿病, 糖尿病 |
详情
|
| Recombinant insulin glargine (Gan & Lee) |
重组甘精胰岛素(甘李药业) |
GL-GLA |
批准上市 |
甘李生物 |
长秀霖, Basalin |
Mainland China |
糖尿病 |
Gan & Lee Pharmaceuticals |
2005-05-10 |
一型糖尿病, 糖尿病 |
详情
|
| Insulin degludec/Insulin aspart |
德谷胰岛素/门冬胰岛素 |
NN-1045; NN-5401; NN-1250/Insulin aspart |
批准上市 |
诺和诺德制药 |
Ryzodeg, 诺和佳 |
Japan |
糖尿病, 一型糖尿病, 二型糖尿病 |
Novo Nordisk A/S |
2012-12-25 |
一型糖尿病, 二型糖尿病, 糖尿病 |
详情
|
| Insulin human (rDNA origin, Novo Nordisk) |
人胰岛素(诺和诺德) |
NN-729 |
批准上市 |
诺和诺德制药 |
Novolin R, 诺和灵R, Mixtard, Actraphane, Insulatard |
United States |
二型糖尿病 |
Novo Nordisk Inc |
1991-06-25 |
一型糖尿病, 二型糖尿病, 戒烟, 高血糖症, 酗酒, 帕金森病, 阿尔茨海默病, 轻度认知障碍, 慢性阻塞性肺疾病, 淋巴瘤, 糖尿病 |
详情
|
| Ceritinib |
色瑞替尼 |
LDK-378; NVP-LDK378; NVP-LDK378-NX |
批准上市 |
诺华制药 |
赞可达, Zykadia |
EU |
非小细胞肺癌 |
Novartis Europharm Ltd |
2014-04-29 |
结直肠癌, 黑色素瘤, 腺癌, 非小细胞肺癌, 甲状腺瘤, 脑转移, 食道腺癌, 浆细胞性肉芽肿, 间变大细胞淋巴瘤, 血液肿瘤, 肝功能衰退, 胆管上皮癌, 胰腺癌, 甲状腺未分化癌, 肿瘤, 胶质母细胞瘤, 胃癌 |
详情
|
| Isophane Protamine Recombinant Human Insulin biosimilar (Popular Pharmaceuticals) |
精蛋白重组人胰岛素生物类似药 (Popular Pharmaceuticals) |
|
批准上市 |
Popular Pharmaceuticals |
Insulin N |
Bangladesh |
糖尿病 |
Popular Pharmaceuticals |
2013-01-01 |
糖尿病 |
详情
|
| Insulin glargine |
甘精胰岛素 |
U-300; Gla-100; Gla-300; Hoe-901; Hoe-71GT; HOE901-U300 |
批准上市 |
赛诺菲 |
来得时, Lantus, Toujeo, Lantus XR, Optisulin |
United States |
一型糖尿病, 二型糖尿病 |
Sanofi-Aventis U.S. Llc |
2000-04-20 |
一型糖尿病, 二型糖尿病, 动脉粥样硬化, 糖尿病酮症酸中毒, 高血糖症, 高胆固醇血症, 高血压, 冠状动脉疾病, 心脏和血管疾病, 糖尿病 |
详情
|
| Insulin human (inhalation powder) |
人胰岛素 |
SAR-439065 |
批准上市 |
Mannkind Corporation |
Technosphere Insulin System, Afrezza, Afresa |
United States |
二型糖尿病 |
Mannkind Corporation |
2014-06-27 |
一型糖尿病, 二型糖尿病, 高血糖症, 哮喘, 慢性阻塞性肺疾病, 糖尿病 |
详情
|
| Insulin human |
|
HR-1799 |
批准上市 |
Sanofi Deutschland |
Insulin Human Winthrop |
EU |
糖尿病, 糖尿病昏迷, 糖尿病酮症酸中毒 |
Sanofi-Aventis Deutschland Gmbh |
1997-02-21 |
糖尿病昏迷, 糖尿病酮症酸中毒, 青光眼, 糖尿病 |
详情
|
| Insulin glargine biosimilar (Incepta Pharmaceuticals) |
甘精胰岛素生物类似药 (Incepta Pharmaceuticals) |
|
批准上市 |
Incepta Pharmaceuticals Ltd |
|
Bangladesh |
一型糖尿病, 二型糖尿病 |
Incepta Pharmaceuticals Ltd |
2012-08-01 |
一型糖尿病, 二型糖尿病 |
详情
|
| Insulin aspart biosimilar (Sanofi) |
门冬胰岛素生物类似药(赛诺菲) |
SAR-341402; SAR-Asp |
批准上市 |
赛诺菲 |
Truvelog |
EU |
糖尿病 |
Sanofi Winthrop Industrie SA |
2020-06-25 |
一型糖尿病, 二型糖尿病, 糖尿病 |
详情
|
| Recombinant lispro insulin biosimilar (Gan & Lee) |
重组赖脯胰岛素生物类似药(甘李药业) |
|
批准上市 |
甘李药业股份有限公司 |
Prandilin, 速秀霖 |
Mainland China |
糖尿病 |
Gan & Lee Pharmaceuticals |
2006-01-19 |
糖尿病 |
详情
|
| Rinsulin NPH (Geropharm) |
|
|
批准上市 |
Geropharm |
Rinsulin NPH |
Russian Federation |
糖尿病 |
Geropharm |
2012-10-01 |
二型糖尿病, 糖尿病 |
详情
|
| Insulin glargine biosimilar (Lilly/Boehringer Ingelheim) |
甘精胰岛素生物类似药 (Lilly/Boehringer Ingelheim) |
LY-2963016; LY2963016-U-100; LY2963016-U-200 |
批准上市 |
Boehringer Ingelheim Gmbh, 礼来制药 |
Basaglar, Abasaglar, Rezvoglar, Abasria |
EU |
糖尿病 |
Eli Lilly Nederland BV |
2014-09-09 |
一型糖尿病, 二型糖尿病, 高血糖症, 慢性肾功能不全, 糖尿病 |
详情
|
| Oral insulin (Generex Biotechnology) |
|
|
批准上市 |
Generex Biotechnology Corp |
Oralin, Oralgen, Oral-lyn, Recosulin |
Ecuador |
一型糖尿病, 二型糖尿病 |
null |
2005-01-01 |
一型糖尿病, 二型糖尿病 |
详情
|
| Insul 30/70 biosimilar (Popular Pharmaceuticals) |
|
|
批准上市 |
Popular Pharmaceuticals |
Insul 30-70 |
|
|
|
|
一型糖尿病, 二型糖尿病, 妊娠期糖尿病 |
详情
|
| Recombinant Human Insulin (Wanbang Pharma) |
重组人胰岛素(万邦生化) |
|
批准上市 |
江苏万邦生化医药集团有限责任公司 |
|
Mainland China |
糖尿病 |
Wanbang Biopharmaceuticals Co Ltd |
2003-08-29 |
糖尿病 |
详情
|
| Insulin degludec |
德谷胰岛素 |
NN-1250; Insulin-454 |
批准上市 |
诺和诺德制药 |
诺和达, Tresiba |
Japan |
二型糖尿病, 糖尿病, 一型糖尿病 |
Novo Nordisk A/S |
2012-09-28 |
一型糖尿病, 二型糖尿病, 糖尿病 |
详情
|
| Insulin glulisine |
谷赖胰岛素 |
HMR-1964 |
批准上市 |
赛诺菲 |
艾倍得, Apidra |
United States |
糖尿病 |
Sanofi-Aventis U.S. Llc |
2004-04-16 |
一型糖尿病, 白血病, 二型糖尿病, 心脏衰竭, 心肌梗塞, 唐氏综合征, 高血糖症, 心脏和血管疾病, 轻度认知障碍, 阿尔茨海默病, 糖尿病, 呼吸功能不全 |
详情
|
| Insulin biosimilar (Julphar) |
胰岛素生物类似药 (Julphar) |
|
批准上市 |
Julphar (Gulf Pharmaceutical Industries) |
Julphar Insulin R |
Tunisia |
糖尿病 |
Julphar (Gulf Pharmaceutical Industries) |
2013-10-01 |
糖尿病 |
详情
|
| Insulin lispro protamine(25R) (Lilly ) |
精蛋白锌重组赖脯胰岛素(25R)(礼来) |
|
批准上市 |
礼来苏州制药有限公司 |
|
|
|
|
|
糖尿病 |
详情
|
| Insulin Aspart 50(Tonghua Dongbao Pharmaceutical) |
门冬胰岛素50(通化东宝) |
|
批准上市 |
通化东宝药业股份有限公司 |
|
Mainland China |
糖尿病 |
Tonghua Dongbao Pharmaceutical Co Ltd |
2022-11-08 |
糖尿病 |
详情
|
| Insulin Aspart 30(onghua Dongbao Pharmaceutical) |
门冬胰岛素30(通化东宝) |
|
批准上市 |
通化东宝药业股份有限公司 |
|
Mainland China |
糖尿病 |
Tonghua Dongbao Pharmaceutical Co Ltd |
2022-11-08 |
糖尿病 |
详情
|
| Recombinant insulin aspart biosimilar (Yichang Hec Changjiang Pharmaceutical) |
门冬胰岛素生物类似药 (宜昌东阳光长江药业) |
|
批准上市 |
宜昌东阳光长江药业股份有限公司 |
|
Mainland China |
糖尿病 |
Yichang Hec Changjiang Pharmaceutical Co Ltd |
2022-10-11 |
糖尿病 |
详情
|
| Insulin Aspart 30 |
门冬胰岛素30 |
|
批准上市 |
诺和诺德制药 |
诺和锐30 |
Mainland China |
糖尿病 |
Novo Nordisk A/S |
2009-09-30 |
糖尿病 |
详情
|
| Isophane Protamine Human Insulin(30R)(Gan & Lee ) |
精蛋白人胰岛素混合注射液(30R)(甘李药业) |
|
批准上市 |
甘李药业股份有限公司 |
|
Mainland China |
糖尿病 |
Gan & Lee Pharmaceuticals |
2021-05-19 |
糖尿病 |
详情
|
| Isophane Protamine Human Insulin(50R)(Zhuhai United Laboratories ) |
精蛋白人胰岛素混合注射液(50R)(珠海联邦制药) |
|
批准上市 |
珠海联邦制药股份有限公司中山分公司 |
诺和灵50R |
Mainland China |
糖尿病 |
Zhongshan Branch of Zhuhai United Laboratories Co Ltd |
2010-12-31 |
糖尿病 |
详情
|
| Insulin human (rDNA) biosimilar (Baxter) |
人胰岛素(rDNA)生物类似药(百特) |
|
批准上市 |
百特国际 |
Inpremzia, Myxredlin |
United States |
糖尿病 |
Baxter Healthcare Corp |
2019-06-20 |
糖尿病 |
详情
|
| Insulin aspart biosimilar (Hisun Pharma) |
门冬胰岛素生物类似药(海正药业) |
|
批准上市 |
浙江海正药业股份有限公司 |
|
Mainland China |
糖尿病 |
Zhejiang Hisun Pharmaceutical Co Ltd |
2021-09-13 |
一型糖尿病, 二型糖尿病, 糖尿病 |
详情
|
| Isophane Protamine Human Insulin(30R)(Hec Pharm) |
精蛋白人胰岛素(30R)(宜昌东阳光) |
|
批准上市 |
宜昌东阳光药业股份有限公司 |
诺和灵30R |
Mainland China |
糖尿病 |
Hec Pharm Co Ltd |
2023-09-19 |
糖尿病 |
详情
|
| Insulin Aspart 30 biosimilar (Jilin Huisheng Biological) |
门冬胰岛素30生物类似药(吉林惠升) |
|
批准上市 |
惠升生物制药股份有限公司 |
|
Mainland China |
糖尿病 |
Jilin Huisheng Biopharmaceutical Co Ltd |
2023-12-26 |
糖尿病 |
详情
|
| Insulin aspart biosimilar(Jilin Huisheng Biological) |
门冬胰岛素生物类似药(吉林惠升) |
|
批准上市 |
惠升生物制药股份有限公司 |
|
Mainland China |
糖尿病 |
Jilin Huisheng Biopharmaceutical Co Ltd |
2023-12-26 |
糖尿病 |
详情
|
| Isophane Protamine Human Insulin(50R)(Novo Nordisk ) |
精蛋白人胰岛素混合注射液(50R)(诺和诺德) |
|
批准上市 |
诺和诺德制药 |
诺和灵50R |
Mainland China |
糖尿病 |
Novo Nordisk (China)Pharmaceuticals Co Ltd |
2019-11-27 |
糖尿病 |
详情
|
| Isophane Protamine Human Insulin(30R)(Novo Nordisk ) |
精蛋白人胰岛素混合注射液(30R)(诺和诺德) |
|
批准上市 |
诺和诺德制药 |
诺和灵30R |
Mainland China |
糖尿病 |
Novo Nordisk A/S |
1999-07-28 |
糖尿病 |
详情
|
| Insulin Aspart 50 biosimilar (Jilin Huisheng Biological) |
门冬胰岛素50生物类似药(吉林惠升) |
|
批准上市 |
惠升生物制药股份有限公司 |
|
Mainland China |
糖尿病 |
Jilin Huisheng Biopharmaceutical Co Ltd |
2023-12-26 |
糖尿病 |
详情
|
| Insulin biosimilar (Harvest Moon Pharmaceuticals) |
胰岛素生物类似药 (Harvest Moon Pharmaceuticals) |
|
批准上市 |
Harvest Moon |
|
|
|
|
|
糖尿病 |
详情
|
| 30/70 Mixture Recombinant Human Insulin(Tonghua Dongbao Pharmaceutical) |
30/70混合重组人胰岛素(通化东宝) |
|
批准上市 |
通化东宝药业股份有限公司 |
甘舒霖30R |
Mainland China |
糖尿病 |
Tonghua Dongbao Pharmaceutical Co Ltd |
2002-05-18 |
糖尿病 |
详情
|
| Mixed Protamine Human Insulin(40R)(Tonghua Dongbao Pharmaceutical) |
精蛋白人胰岛素(40R)(通化东宝) |
|
批准上市 |
通化东宝药业股份有限公司 |
甘舒霖N |
Mainland China |
糖尿病 |
Tonghua Dongbao Pharmaceutical Co Ltd |
1999-01-01 |
糖尿病 |
详情
|
| 50/50 Mixture Recombinant Human Insulin(Tonghua Dongbao Pharmaceutical) |
50/50混合重组人胰岛素(通化东宝) |
|
批准上市 |
通化东宝药业股份有限公司 |
甘舒霖50R |
Mainland China |
糖尿病 |
Tonghua Dongbao Pharmaceutical Co Ltd |
2008-02-04 |
糖尿病 |
详情
|
| Insulin Aspart 50 |
门冬胰岛素50 |
|
批准上市 |
诺和诺德制药 |
诺和锐50 |
Mainland China |
糖尿病 |
Novo Nordisk A/S |
2012-02-10 |
糖尿病 |
详情
|
| Insulin aspart biosimilar (Gan & Lee) |
门冬胰岛素生物类似药(甘李生物) |
GL-ASP |
批准上市 |
甘李生物 |
Rapilin®30, Rapilin30, 锐秀霖30 |
Mainland China |
一型糖尿病, 二型糖尿病 |
Gan & Lee Biotech |
2020-06-05 |
一型糖尿病, 二型糖尿病, 糖尿病 |
详情
|
| Isophane Protamine Human Insulin(30R)(Lilly) |
精蛋白人胰岛素(30R)(礼来) |
|
批准上市 |
礼来制药 |
|
Mainland China |
糖尿病 |
Eli Lilly And Company |
|
糖尿病 |
详情
|
| Insulin lispro protamine biosimilar(25R) (Gan & Lee) |
精蛋白锌重组赖脯胰岛素生物类似药(25R)(甘李药业) |
|
批准上市 |
甘李生物 |
Prandilin25, 速秀霖25 |
Mainland China |
糖尿病 |
Gan & Lee Pharmaceuticals |
2014-05-09 |
糖尿病 |
详情
|
| Insulin lispro |
赖脯胰岛素 |
LY-900027; LY-275585; Lys-B28; Pro-B29; LYSPRO; LY-900014 |
批准上市 |
礼来制药 |
Humalog, 优泌乐, Eglucent, Liprolog, Lyumjev, Liumjev |
EU |
糖尿病 |
Eli Lilly Nederland BV |
1996-04-30 |
一型糖尿病, 二型糖尿病, 胰岛素抵抗, 糖尿病神经病变, 糖尿病, 高脂血症 |
详情
|
| Recombinant human insulin (Biocon) |
|
|
批准上市 |
Biocon Ltd |
Insugen |
India |
糖尿病 |
Biocon Ltd |
2004-11-10 |
糖尿病 |
详情
|
| Isophane Protamine Human Insulin(40R)(Tonghua Dongbao) |
精蛋白人胰岛素混合注射液(40R)(通化东宝药业股份有限公司) |
|
批准上市 |
通化东宝药业股份有限公司 |
|
|
|
|
|
糖尿病 |
详情
|
| Insulin glargine biosimilar (Zhuhai United) |
甘精胰岛素生物类似药 (珠海联邦) |
|
批准上市 |
珠海联邦制药股份有限公司 |
|
Mainland China |
糖尿病 |
Zhuhai United Laboratories Co Ltd |
2016-12-23 |
糖尿病 |
详情
|
| Isophane Insulin(Polish Society of Diabetology) |
低精蛋白锌胰岛素(Polish Society of Diabetology) |
|
批准上市 |
Polish Society of Diabetology |
|
|
|
|
|
糖尿病 |
详情
|
| Insulin human(Organon France) |
人胰岛素(Organon France) |
|
批准上市 |
Organon France |
|
EU |
糖尿病 |
Organon France |
1999-11-03 |
糖尿病 |
详情
|
| Isophane Protamine Recombinant Human Insulin(Novo Nordisk) |
精蛋白重组人胰岛素(诺和诺德) |
|
批准上市 |
诺和诺德(中国)制药有限公司 |
诺和灵N |
Mainland China |
糖尿病 |
Novo Nordisk (China)Pharmaceuticals Co Ltd |
2016-06-16 |
糖尿病 |
详情
|
| Isophane Protamine Recombinant Human Insulin (BIOTON) |
精蛋白重组人胰岛素(BIOTON) |
|
批准上市 |
Bioton Sa |
|
Mainland China |
糖尿病 |
Bioton Sa |
2013-03-29 |
糖尿病 |
详情
|
| Insulin Glargine biosimilar (Liaoning Bo'Ao Bio-Pharmaceutical) |
甘精胰岛素生物类似药(辽宁博鳌生物制药) |
|
批准上市 |
辽宁博鳌生物制药有限公司 |
|
Mainland China |
糖尿病 |
Liaoning Bo'Ao Bio-Pharmaceutical Co Ltd |
2023-12-05 |
二型糖尿病, 糖尿病 |
详情
|
| Insulin Aspart 30 (Yichang Hec Changjiang Pharmaceutical) |
门冬胰岛素30(宜昌东阳光长江药业) |
|
批准上市 |
宜昌东阳光长江药业股份有限公司 |
|
Mainland China |
糖尿病 |
Yichang Hec Changjiang Pharmaceutical Co Ltd |
2022-11-01 |
二型糖尿病, 糖尿病 |
详情
|
| Isophane Protamine Recombinant Human Insulin (Hefei Tianmai Biotechnology) |
精蛋白重组人胰岛素(合肥天麦生物) |
|
批准上市 |
合肥天麦生物科技发展有限公司 |
|
Mainland China |
糖尿病 |
Hefei Tianmai Biotechnology Development Co Ltd |
2019-09-05 |
一型糖尿病, 二型糖尿病, 糖尿病 |
详情
|
| Recombinant human insulin (Hefei Tianmai Biotechnology) |
重组人胰岛素(合肥天麦生物) |
|
批准上市 |
合肥天麦生物科技发展有限公司 |
|
Mainland China |
糖尿病 |
Hefei Tianmai Biotechnology Development Co Ltd |
2018-04-28 |
糖尿病 |
详情
|
| Lixisenatide/Insulin glargine |
利司那肽/甘精胰岛素 |
HOE-901/AVE-0010 |
批准上市 |
赛诺菲 |
Soliqua, iGlarLixi, Suliqua |
United States |
二型糖尿病 |
Sanofi-Aventis U.S. Llc |
2016-11-21 |
一型糖尿病, 二型糖尿病 |
详情
|
| Insulin icodec |
依柯胰岛素 |
148-0287-A; LAI-287; Long-acting basal insulin analogue; NN-1436; Insulin-287; NNC-0148-0000-0287; NNC0148-0287 C; OI-287GT; NN-1956 |
批准上市 |
诺和诺德制药 |
AWIQLI, Awiqli |
Canada |
糖尿病 |
Novo Nordisk Canada Inc |
2024-03-12 |
一型糖尿病, 二型糖尿病, 糖尿病 |
详情
|
| Insulin lispro biosimilar (Wanbang Biopharma) |
重组赖脯胰岛素生物类似药(万邦医药) |
|
批准上市 |
江苏万邦生化医药集团有限责任公司 |
|
Mainland China |
糖尿病 |
Wanbang Biopharmaceuticals Co Ltd |
2022-01-25 |
糖尿病 |
详情
|
| Recombinant human insulin (Shenzhen Kexing Biotech) |
重组人胰岛素注射液 |
|
批准上市 |
深圳科兴生物工程有限公司 |
苏泌啉, Su Bi Lin |
Mainland China |
糖尿病 |
Shenzhen Kexing Biological Engineering Co Ltd |
2002-06-10 |
糖尿病 |
详情
|
| Recombinant insulin (Horizon Pharma/Ibatech) |
|
|
批准上市 |
Savient Pharmaceuticals Inc |
|
Poland |
一型糖尿病 |
null |
2002-07-01 |
一型糖尿病 |
详情
|
| Rosinsulin |
|
|
批准上市 |
Medsintez |
|
|
|
|
|
糖尿病 |
详情
|
| Isophane Insulin |
低精蛋白锌胰岛素 |
|
批准上市 |
江苏万邦生化医药集团有限责任公司 |
万苏林 |
Mainland China |
糖尿病 |
Wanbang Biopharmaceuticals Co Ltd |
1997-01-01 |
糖尿病 |
详情
|
| Recombinant human insulin (Incepta) |
重组人胰岛素(Incepta) |
|
批准上市 |
Incepta Pharmaceuticals Ltd |
Maxsulin |
Bangladesh |
糖尿病 |
Incepta Pharmaceuticals Ltd |
2012-01-01 |
糖尿病 |
详情
|
| Recombinant human insulin (SciGen) |
|
|
批准上市 |
Generex Biotechnology Corp |
Recojet |
India |
糖尿病 |
null |
2004-12-01 |
糖尿病 |
详情
|
| Insulin aspart biosimilar (Zhuhai United Laboratories) |
门冬胰岛素生物类似药(珠海联邦制药) |
|
批准上市 |
珠海联邦制药股份有限公司 |
联邦优倍灵, Ublin |
Mainland China |
糖尿病 |
Zhuhai United Laboratories Co Ltd |
2021-07-12 |
糖尿病 |
详情
|
| Recombinant human insulin (HEC Pharm) |
重组人胰岛素 (东阳光) |
|
批准上市 |
宜昌东阳光长江药业股份有限公司 |
|
Mainland China |
糖尿病 |
Yichang Hec Changjiang Pharmaceutical Co Ltd |
2020-06-05 |
糖尿病 |
详情
|























 Star Ribbon预染蛋白Marker蛋白质标记物是生物研究和药物开发的重要组成部分。无论是用于蛋白质电泳还是western blot,我们的预染色蛋白质标记物帮助您快速确定目标蛋白质的分子量或评估转移效率。Fc受体蛋白治疗性抗体的功效取决于Fab片段及其对目标抗原的结合活性,还取决于Fc片段及其与关键Fc受体的相互作用。因此,在抗体工程中候选物必须针对一系列受体进行测试。探索我们的重组Fc受体蛋白质的全面收藏!
Star Ribbon预染蛋白Marker蛋白质标记物是生物研究和药物开发的重要组成部分。无论是用于蛋白质电泳还是western blot,我们的预染色蛋白质标记物帮助您快速确定目标蛋白质的分子量或评估转移效率。Fc受体蛋白治疗性抗体的功效取决于Fab片段及其对目标抗原的结合活性,还取决于Fc片段及其与关键Fc受体的相互作用。因此,在抗体工程中候选物必须针对一系列受体进行测试。探索我们的重组Fc受体蛋白质的全面收藏!






























 膜杰作
膜杰作 Star Staining
Star Staining